Meaningful Evidence
Nevro HFX’s unique specific FDA indication is the outgrowth of years of investments in clinical trials culminating with the SENZA-NSRBP, the first and only RCT evaluating SCS for this specific patient population to date.


The SCS system approved by the FDA with a specific indication for Non-Surgical Back Pain.
Nevro HFX’s unique specific FDA indication is the outgrowth of years of investments in clinical trials culminating with the SENZA-NSRBP, the first and only RCT evaluating SCS for this specific patient population to date.
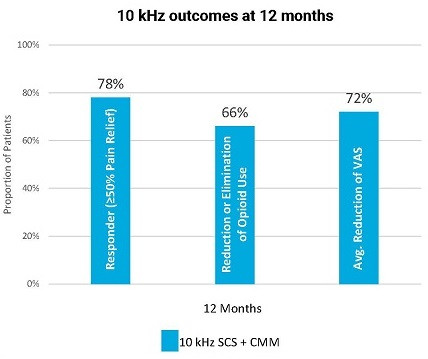
Within the SENZA-NSRBP, 10 kHz Therapy
demonstrated significant and durable outcomes across
responder rates, functional improvements and opioid use
as compared to CMM alone. There were no explants due
to loss of efficacy.
Direct Neural Inhibition With 10 kHz Therapy

Historically, non-surgical back pain patients have struggled with lack of treatment options when CMM fails. Outcomes demonstrated in the SENZA-RCT were a result of the use of 10 kHz Therapy and it’s unique mechanism of action that only Nevro HFX can deliver.