Meaningful Evidence
Nevro’s unique PDN indication is the outgrowth of years of investments in clinical trials culminating with the SENZA-PDN, the largest RCT evaluating SCS for PDN to date.


The SCS system approved by the FDA with a specific indication for Painful Diabetic Neuropathy.
Nevro’s unique PDN indication is the outgrowth of years of investments in clinical trials culminating with the SENZA-PDN, the largest RCT evaluating SCS for PDN to date.
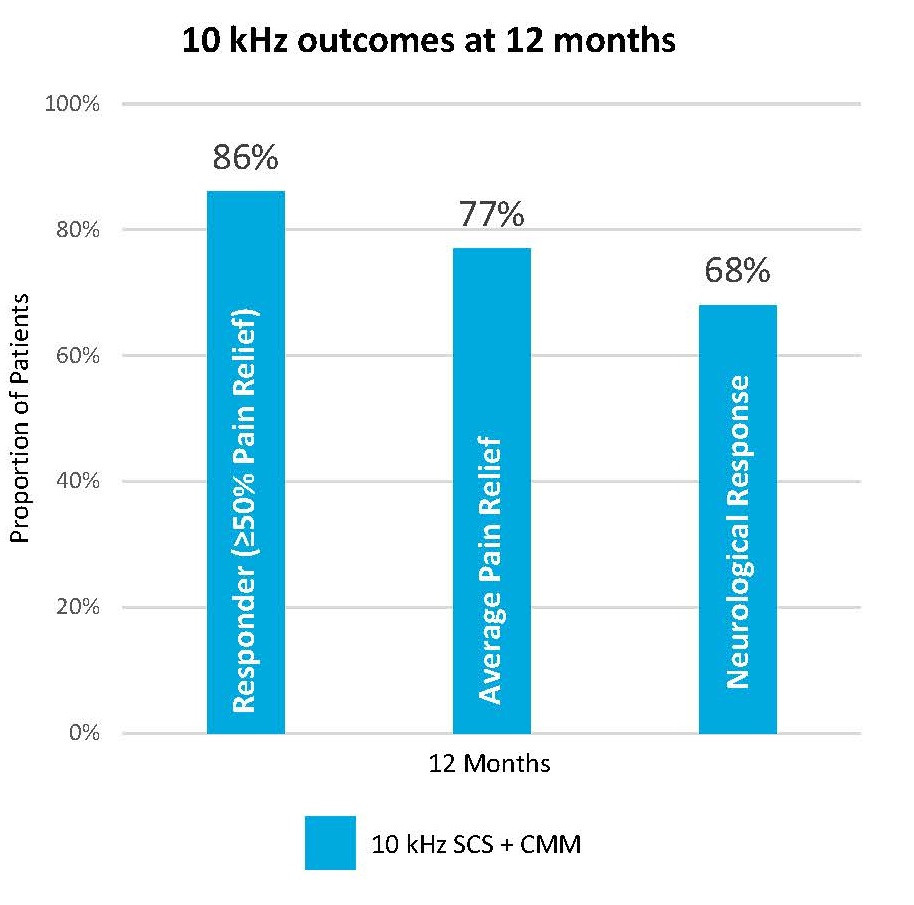
Within the SENZA-PDN, 10 kHz Therapy demonstrated exceptional outcomes across responder rates, pain relief, and even sensory response.
Direct Neural Inhibition With
10 kHz Therapy

Historically, PDN patients have struggled with a lack of treatment options when CMM fails. Now, 10 kHz Therapy and a unique mechanism of action offer new potential for relief.